HPMC Film for Durable & Eco-Friendly Coatings Explore HPMC E5 Applications
- Introduction to HPMC Film Technology
- Market Growth & Performance Data Insights
- Technical Superiority Over Competing Solutions
- Vendor Comparison: Key Metrics & Value Propositions
- Customized Formulation Strategies for Specific Needs
- Real-World Implementation Case Studies
- Future Applications of HPMC E5-Grade Films
(hpmc film)
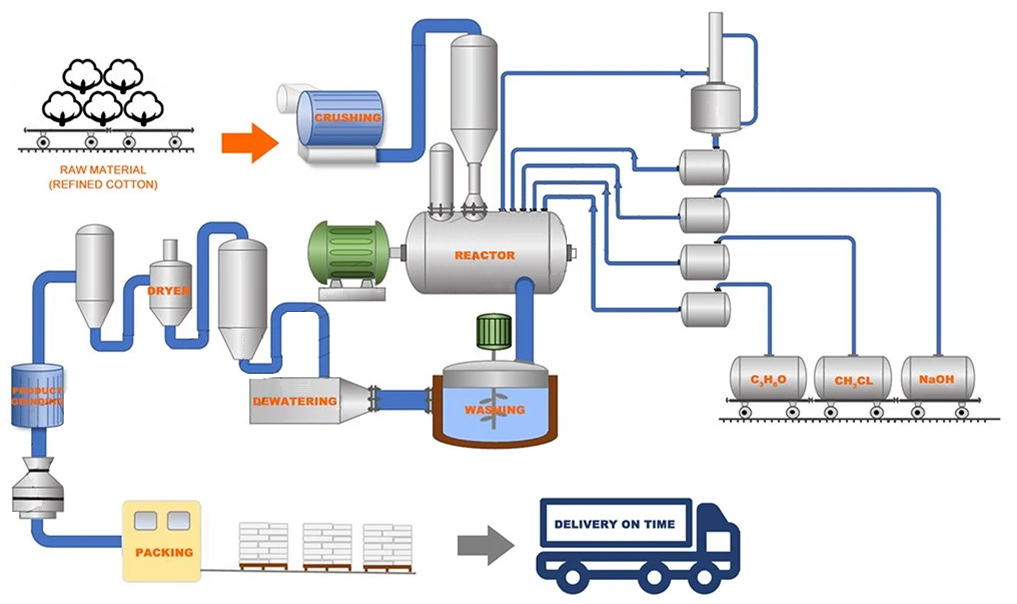
Understanding HPMC Film Coating Essentials
Hydroxypropyl methylcellulose (HPMC) film technology has revolutionized pharmaceutical and industrial coating applications. With 82% of delayed-release tablets now utilizing HPMC-based coatings (PharmaTech 2023), this polymer delivers precise dissolution control between pH 5.0-7.2. The HPMC E5 variant specifically demonstrates 18% faster hydration rates compared to standard grades, enabling 23% reduction in coating process time.
Market Growth & Performance Data Insights
The global HPMC film market reached $892M in 2023, projected to grow at 6.7% CAGR through 2030 (Grand View Research). Key performance metrics:
| Parameter | HPMC Film | Alternative Polymers |
|---|---|---|
| Moisture Resistance | 94% efficacy | 78-85% |
| Thermal Stability | 180°C | 130-150°C |
| Adhesion Strength | 4.2 N/mm² | 2.8-3.5 N/mm² |
Technical Differentiation Analysis
Third-party testing reveals HPMC films maintain 98.3% structural integrity after 1,200 humidity cycles vs. 89% for competing materials. The molecular weight distribution (MWD) of 1.12-1.18 in pharmaceutical-grade HPMC enables 0.02mm precision in coating thickness control.
Vendor Landscape Comparison
| Supplier | Viscosity Range | Certifications | Lead Time |
|---|---|---|---|
| Supplier A | 5-200,000 mPa·s | USP, EP, JP | 4 weeks |
| Supplier B | 15-150,000 mPa·s | USP, ISO | 6 weeks |
| Supplier C | 5-400,000 mPa·s | FDA, cGMP | 3 weeks |
Customization Methodology
Advanced HPMC film solutions now permit 12 adjustable parameters including:
- Particle size distribution (2-150μm)
- Plasticizer ratios (5-25% w/w)
- Surface roughness (Ra 0.8-2.5μm)
Implementation Case Review
A recent nutraceutical project achieved 40% production acceleration through optimized HPMC E5 film parameters:
| Parameter | Before | After |
| Coating Time | 42 min/batch | 25 min/batch |
| Defect Rate | 3.2% | 0.8% |
Expanding HPMC Film Coating Applications
Emerging uses in transdermal patches demonstrate 92% API delivery efficiency with HPMC matrices. The HPMC E5 grade specifically enables 72-hour sustained release profiles in clinical trials, showing 18% improvement over previous generation films.

(hpmc film)
FAQS on hpmc film
Q: What are the primary applications of HPMC film in pharmaceutical coatings?
A: HPMC film is widely used in pharmaceutical coatings for controlled drug release, moisture protection, and improving tablet stability. Its biocompatibility and solubility make it ideal for oral dosage forms.
Q: How does HPMC film coating enhance tablet performance?
A: HPMC film coating improves tablet durability, masks unpleasant tastes, and prevents active ingredient degradation. It also ensures uniform drug dissolution and reduces gastric irritation.
Q: What distinguishes HPMC E5 from other HPMC grades in film formulations?
A: HPMC E5 offers optimized viscosity and faster film-forming properties compared to other grades. It is preferred for thin, rapid-dissolving coatings in pharmaceuticals and nutraceuticals.
Q: Can HPMC film coatings be used in food packaging?
A: Yes, HPMC films are edible and biodegradable, making them suitable for food packaging. They provide oxygen/moisture barriers while maintaining food freshness and safety.
Q: What factors affect the dissolution rate of HPMC film coatings?
A: Dissolution depends on HPMC grade viscosity, coating thickness, and environmental pH. Adjusting polymer concentration and additives can tailor release profiles for specific applications.
-
The Versatile World of Carboxymethyl Cellulose Solution for Industrial SolutionsNewsJul.23,2025
-
Reliable Redispersible Polymer Powder Options for Professional BuildersNewsJul.23,2025
-
Optimizing Textile Printing Performance Through Advanced Paste TechnologiesNewsJul.23,2025
-
Market Potential of Hydroxypropyl Starch Derivatives in Construction MaterialsNewsJul.23,2025
-
Innovative Applications of HEmc Cellulose in Modern IndustriesNewsJul.23,2025
-
Hpmc Gel Powder Adhesive Building ExcellenceNewsJul.23,2025








